We are bringing research to new communities and expanding access to care.
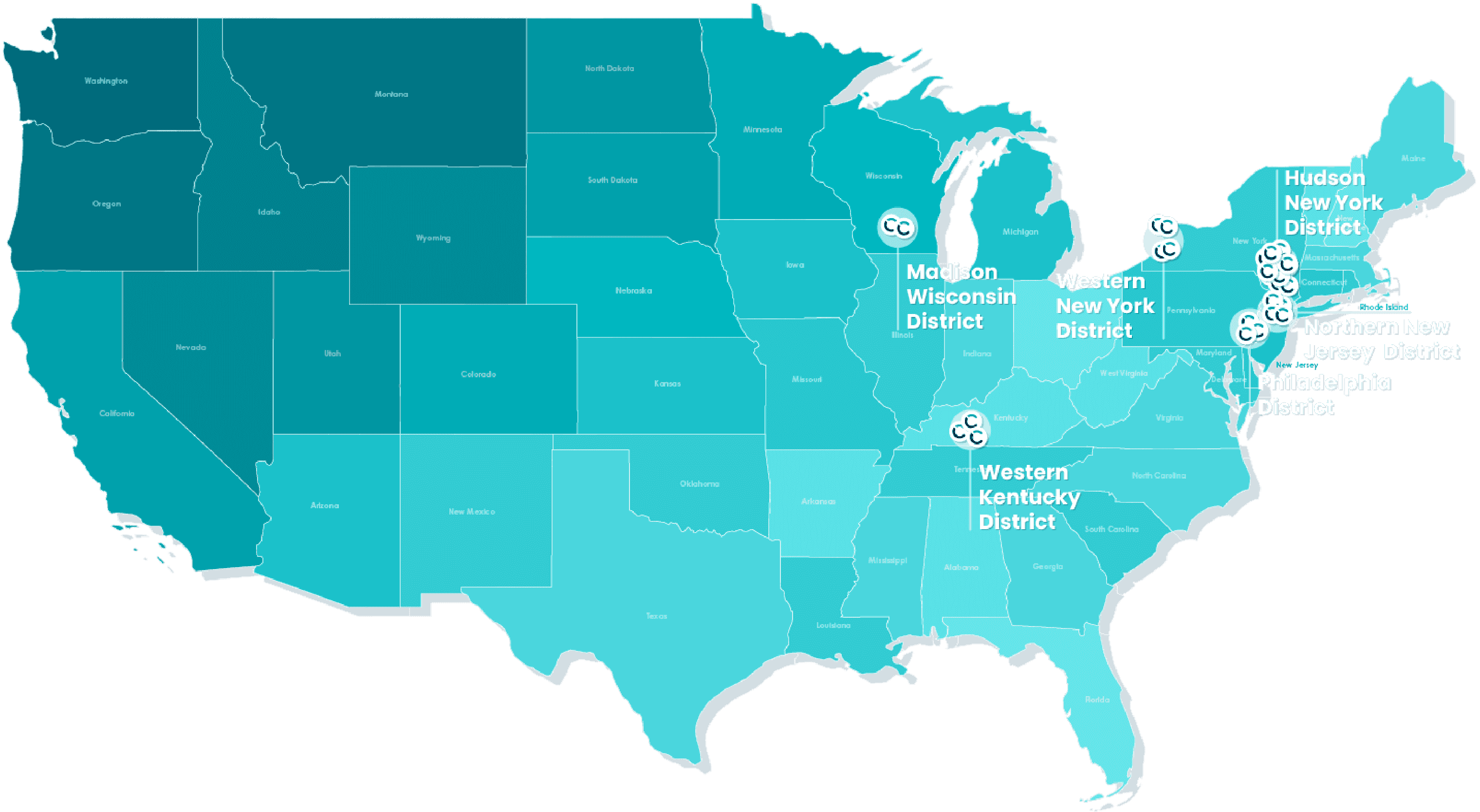
The Site Partnership Network supports traditional site models with an embedded team of experienced research professionals at sites within community healthcare reaching diverse patient populations that are less saturated.
Site Partnership Network
We provide an extensive and diverse funnel of trial opportunities to investigators directly from our partners, CROs, and sponsors, effectively bringing research into communities where care is provided by the health care providers they trust most. Where access to clinical research typically isn’t available, we’re equipped to bring trial opportunities as a care option in historically underrepresented communities.
Therapeutic Areas of Interest
- Gastroenterology
- Rheumatology
- Respiratory
- Cardiology
- Pulmonology
- Endocrinology
- Infectious Disease
- Oncology
- Nephrology
- Immunology
Key Indications
- Afib/CAD/HF
- Hypertension
- UC/Crohns/IBD
- Depression/Anxiety
- Type 2 Diabetes
- COPD/Asthma
- PsA/Lupus/RA
- Obesity
- Cancer
- CKD
- Stroke
- Vaccine
Features
Testimonials
[We are] proud to partner with Circuit Clinical to manage our Phase I study for the company's premier product…
this product is a food allergy treatment for peanut allergy delivered via a specially formulated toothpaste designed to optimize exposure of allergenic proteins to a patient's immune system while also cleaning their teeth."
Small-Sized Biotech
Our relationship with Circuit Clinical is built on history, transparency, and innate trust.
Most recently they helped us capture [COVID-19] data at an intense speed and knowing we could count on them to do so only added to the efficiency. As dedicated partners we've achieved hockey-stick-like growth—our joint and promising future together is guaranteed."
Mid-Sized Biotech
Featured Case Study
Ongoing CMV Vaccine Study Exceeding Enrollment Goals
A Phase 1/2, First-in-Human (FIH), randomized, observer-blind, placebo-controlled, dose-escalation study to assess the safety, reactogenicity, and immunogenicity of a candidate cytomegalovirus (CMV) vaccine comprising recombinant protein and adjuvant when administered in healthy adults. Circuit partnered with a Top 10 pharma company that is a leader in vaccine development to conduct their Phase 1/2 CMV vaccine study in one of our community research sites.
Featured Case Study
Specialized Patient Recruitment Led to Strong Vaccine Study Enrollment
A phase 2/3, randomized, observer-blind, placebo-controlled study to evaluate the efficacy and safety of an investigational vaccine targeting respiratory syncytial virus (RSV) in adults ≥60 years of age. Circuit Clinical partnered with an industry-leading biotechnology company to enroll patients in their phase 2/3 RSV vaccine study. This study was placed in one of our Community Research Network sites with extensive experience in non-COVID vaccines.
Want to learn more about our Site Partnership Network?
Contact our team to discuss our solutions for clinical research or read more of our Site Partnership Network case studies.
Learn more about how we support virtual trials with our Remote Site Network, part of our Community Research Network.
Remote Site Network


